ACTG-CRO (Africa Clinical Trials Group) is a Full-Service Contract Research Organization (CRO) based in France & Tunisia that provides Clinical Trials and Pharmaceutical Services at International Standards to local and international Pharmaceutical companies, Biotech, major CROs, research institutions and other health related organization, seeking to conduct clinical trials in Africa or in Europe.

ACTG-CRO is a privately held company composed of 3 departments:
- CRO services Department: Sites & Investigators Selection, Clinical Trials Monitoring, Remote Centralized Monitoring, SMO, Outsourcing Staff, Bioequivalence Studies (BE Center is WHO certified), Biostatistics…
- Pharmaceutical Services Department: Among our advantages, we are expert in registering Plant in Libya or preparing the dossiers for submission to obtain MA authorization in any African countries: Pharmaceutical Drug and Devices Registration with Market Authorization or Plant Registration in Libya, Product Representation, Marketing, Medical Promotion and Distribution.
- Training Department: CF ACTG is the Training Center of ACTG-CRO (CRAs, Medical Representatives, Investigators…) with a Ministry Agreement and registered on Transcelerate Biopharma as a GCP-Provider.
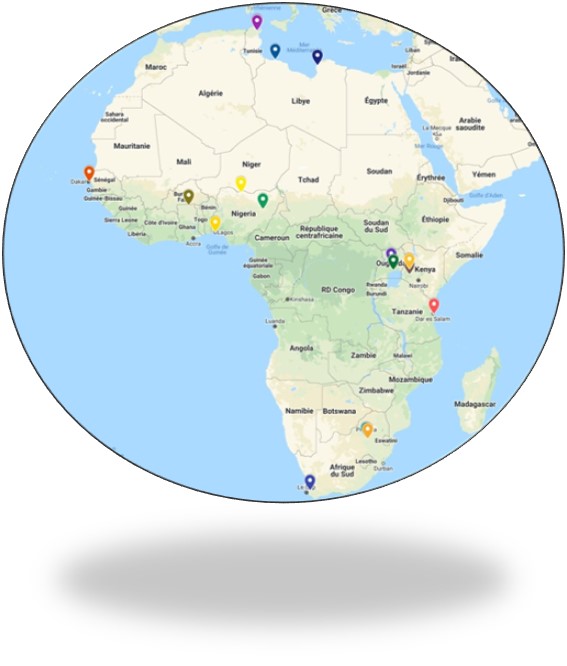
ACTG-CRO operates in a number of countries in Africa and have the capability and expertise to conduct clinical trials and development projects on both a local, cross-region and global basis and provide the full range of ACTG-CRO Services.
ACTG-CRO is registered in France as a CRO services provider, Training, and having also a drug brokerage activity registered at ANSM (National Agency for the Safety of Medicines and Health Products). Thus, ACTG-CRO adheres to EU regulations and standards, ensuring high levels of compliance, data protetcion, and quality assurance.
This dual presence of ACTG-CRO, in Europe and in Africa, offers European-quality service at a more affordable rate, providing significant cost saving.
OUR MISSION & OUR VALUE

ACTG-CRO is committed to implementing International Standards in Clinical Trials through Training
To improve health and to improve life in African countries, by improving access to healthcare through clinical studies. We deliver what we promise with honesty and integrity, thus earning the trust that fosters long term relationships.
To provide professional, cost-effective and timely assistance to our Clients to contribute to their drug development efforts and to guarantee the collection of high-quality clinical data in Africa in compliance with international standards and local regulations that warrant subject protection.

ACTG-CRO has formed strategic development partnerships in Africa with a list of investigators experienced in clinical trials
Our goal is to improve access to healthcare for patients in Africa
Healthcare Equity: Clinical trials can bring advanced medical treatments and interventions to regions that may otherwise lack access to cutting-edge healthcare. This helps bridge the gap between healthcare standards in Africa and other parts of the world.
Disease Burden: Africa faces a high burden of infectious diseases like malaria, HIV/AIDS, and tuberculosis, as well as rising cases of non-communicable diseases such as diabetes, hypertension and Cancer. Clinical trials focused on these conditions can lead to the development of effective treatments and preventive measures tailored to the unique needs of the African population.
Local Relevance: Conducting clinical trials in Africa ensures that the data collected is relevant to the local population. Genetic, environmental, and lifestyle differences can affect how diseases manifest and how patients respond to treatments. Clinical Trials conducted locally can produce more accurate and applicable results.
Capacity Building: Engaging in clinical trials helps build local healthcare infrastructure, including training healthcare professionals, improving laboratory facilities, and developing regulatory frameworks. This enhances the overall healthcare system’s capability to manage various health issues effectively.
Economic Benefits: Clinical trials can stimulate economic growth by creating jobs, fostering research and development, and attracting investments from pharmaceutical companies and global health organizations. This economic boost can indirectly improve healthcare access and quality.
Trust and Participation: Including African populations in global clinical research can build trust between communities and healthcare providers. When people see that trials are conducted ethically and transparently and that they benefit from them, they are more likely to participate in future research and healthcare initiatives.
Global Health Security: Addressing health issues in Africa through clinical trials can have global implications. Diseases do not respect borders, and improving health outcomes in Africa can contribute to global efforts to control and eradicate infectious diseases enhancing global health security, and to strive to let cancer patients to benefit from early supportive care.

Clinical trials can significantly improve healthcare access and outcomes in Africa, leading to healthier populations and more robust healthcare systems
OUR VISION

To become the leading experts in clinical trials in Africa and Pharmaceutical Services due to our quality services, the guarantee of study subject protection and establishing long-term trusting relationships with our Partners and Clients.
OUR ADVANTAGES


With our local presence in African countries, clients are guaranteed that their trials will be conducted according to international standards, having a base in France, ACTG-CRO ensures familiarity with European market standards, regulations and businesses practices, providing a bridge between Tunisian technical expertise and European business requirements.
- Strategic location: Tunisia’s location in North Africa, close to Europe, allows for convenient travel and communication. It also offers a startegic position for businesses looking to expand into African or Middle Eastern countries.
- Skilled Workforce in Tunisia: High level of expertise and education found in Tunisia.
- Cost Efficiency: Overheads and Salaries are generally lower than in Europe. This can translate to more competitive pricing for the European company without compromising on quality.
We assign locally-based CRAs trained to clinical trials regulations and SOPs (both company and sponsor SOPs). The fact we run operations from Tunisia, apart from Europe, US enables us to provide very competitive rates. We provide you Clinical Sites to implement your trial.

With our Expertise in clinical trials and our list of Experienced Investigators specialized in a range of therapeutic areas with whom we entertain great relationships, we are ensuring to provide the services you need with the excellent process.

We master all the local processes for documents submission. We work closely and cooperating with the Ministry of Health of Tunisia, Algeria and Libya. We collaborate with our Partners in Senegal and Ivory Coast.

We have comprehensive and detailed SOPs to guide and train all the staff, to ensure operations to be carried out in compliance with ICH/GCP recommendations and local regulations, and to fulfill Sponsor requests and requirements.
